
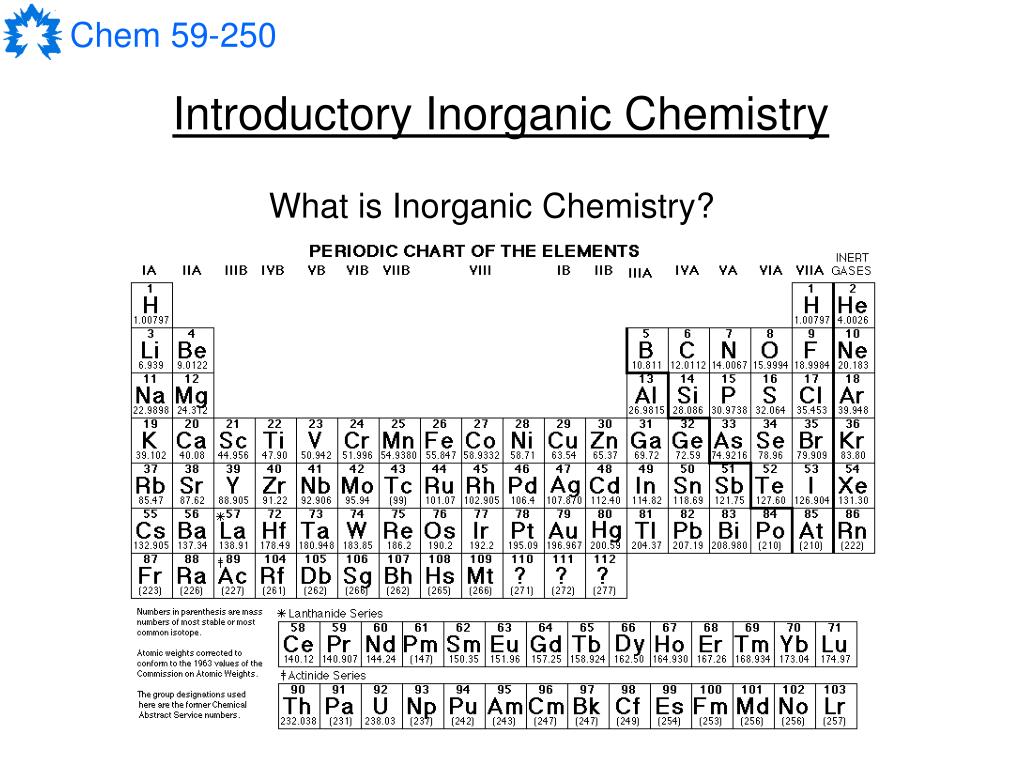
The transitional metals fall nicely between alkali earths and poor metals and metalloids - see for an explanation. The atomic radii are fairly close for the lanthanides and actinides. For example, the lanthanides tend to form 3+ valences, and so the oxides are generally M 2O 3. They are grouped however for reasons of similarity in some chemical properties. But the main idea is that there are alternate forms that do not 'break out' the lathanides and actinides. This website - chemlab.pc./periodic/periodic.html - has alternative forms of the periodic table, however there seems to be some problem with it at the time this is posted. is there any reason that they broke both the lanthanides and actinides from the periodic table? The weight percent of oxygen in an oxide that has the formula MO2, is 15.2%.I have a question about lanthanides and actinides. The atomic mass of an element can be used to convert Avogadro’s number can be used to convert the atomic mass of an element to the mass of one mole of that element. How does Avogadro’s number relate to the atomic mass and moles of an element? 1. The easiest fusion reaction to initiate is 1-1H + 1-1H-> 4-2He + 1-0 n The 1-1 indicates a small one above the other on the left side of the symbol of the element.So for 1-0n is a one above a zero on the left side of n (neutron).

calculate the atomic mass of element X given the abundance of X-7 is 92.5%. The average atomic mass of iridium is 192.22u, if iridium-191 has an atomic mass of 190.961u and an isotopic abundance of 37.3% and iridium-193 is the only other natural occuring isotope, what is the atomic mass of iridium-193? I tried but I don't think IĮlement X has two natural isotopes:X-6 (6.015amu) and X-7 (7.016amu). because neutrons determine an atom's chemical properties, isotopes of the same element are very Because isotopes have different numbers of neutrons, they have different mass numbers. Which of the following statements about isotopes is/are true? a. The electronic configuration of an element X is: 1s² 2s² 2p6 3s2 3p5 Deduce the atomic number of X To what group does it belong Give two properties of the group to which the element X belong. Which of the following represents a pair of isotopes? Atomic Number Mass Number a. Using the atomic mass reported on the periodic table, determine the mass of bromine-81, the other isotope of bromine. Bromine-79 has a mass of 78.918 amu and is 50.69% abundant.

with mass numbers 191 and 193, and its average relative atomic mass is 192.23.calculate the relative abundances of the two isotopesīromine has two naturally occurring isotopes. What is the percent abundance of 153 Eu ? The atomic mass given for Eu is 151.9640 u. am i supposed to multiply theĮuropium has two stable isotopes, 151 Eu (150.9198 u) and 153 Eu (152.9212 u). Rhenium is 62.60% 187Re, and the atomic mass of 187Re is 186.956 amu. The element rhenium (Re) has two naturally occurring isotopes, 185Re and 187Re, with an average atomic mass of 186.207 amu. Within these or other sites, you can use the command with key words to find information more quickly. To answer your questions, you can do other web searches with the appropriate key words. Background assumed: This course will use your undergraduate. Since this is not my area of expertise, I searched Google under the key words " periodic table" to get these possible sources: I'll be glad to help but I don't want to simply answer each of them. The site is provided by the Chemlab server of Phoenix College, AZ. Your notes/book should provide insight into most of these. Graphs and tables of element properties, alternative styles of periodic table (e.g., spiral, pyramid), a special page on isotopic properties, a printable table, and links to other periodic table pages are among the wealth of information provided. I would be interested in hearing what you think or what you have found about each of these questions. Why is hydrogen, h, most often considered a nonmetallic element? Are most elements metallic or nonmetallic?ħ. Jean-Claude Risset: An introductory catalog of computer synthesized sounds. How is the periodic table more than just a listing of the know elements?Ħ. Distinguish between mass number and atomic mass.ĥ. This site provides access to resources he developed along with students and colleagues that remain somewhat useful. Distinguish between atomic number and mass number.Ĥ. Brooks separated from the University of Nebraska-Lincoln on August 15, 2015, under the Voluntary Separation Incentive Program. 1.What effect do isotopes of a given element have on the atomic mass calculated for that element?ģ. In the periodic table atomic mass number that we see is an average of all the.


 0 kommentar(er)
0 kommentar(er)
